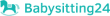Salaire moyen: CHF120 105 /annuel
Plus de statistiquesRecevoir les offres d'emploi par email
- ...As a Principal Medical Writer you will provide communication expertise and lead a strategy-driven approach to authoring of key clinical and regulatory documents and regulatory submissions to ensure clarity credibility and consistency of clinical information throughout...SuggéréTemps pleinRemote job
- ...positive impact to help ultra-rare disease patients who are in need of life saving treatments Job Description As a Principal Medical Writer, you will provide communication expertise and lead a strategy-driven approach to authoring of key clinical and regulatory...SuggéréTemps plein
- ...patients who are in need of life saving treatments Job Description The ML will be responsible for driving strategic country medical activities in Switzerland in alignment with regional initiatives and the Global Medical Plan. The ML will proactively manage...SuggéréTemps plein
- ...disease, including inflammatory bowel disease, given its substantial individual and societal impact. The Opportunity As a Medical Director, Immunology in Immunology Early Development, you will use your immunology and clinical expertise to help advance the...SuggéréTemps pleinHoraire flexible
- ...Included but not limited to: Build and execute the Global Medical strategy including but not limited to the following deliverables: Build and execute global medical strategy and tactical plan Manage engagement of global KOLs in the therapeutic area Give input...SuggéréTemps pleinRemote jobAssurance-invaliditéHoraire flexible
- ...The Medical Excellence Manager is responsible for managing Investigator Sponsored Studies (ISS) Managed Access Programs (MAP) and Post Trial Access (PTA) Programs. Key responsibilities include: Oversee the ISS review and approval process including proposal validation...SuggéréTemps pleinRemote job
- ...cancers harboring mutations in the RAS signaling pathway. The Opportunity: Reporting to the VP GM Midsized Markets the Senior Medical Director Midsize Markets Europe is the medical leader responsible for defining and executing the medical strategy for oncology...SuggéréTemps pleinRemote job
- ...The Associate Director Medical Affairs Tryngolza sits within the global Tryngolza medical affairs and clinical development (MACD) team and is responsible for supporting the execution of the global medical plan for the assigned product in collaboration with the rest of...SuggéréTemps pleinRemote job
- ...activities in cross-functional teams for easy-to-use, safe and robust products Participate to the complete development process of medical devices (ideation, brainstorming, prototyping, piloting and transfer to manufacturing) Create and review IP Create, manage and...Suggéré
- ...Job Description Summary The Director Medical AI & Innovation is accountable for translating key medical challenges into scalable innovation opportunities solved by AI including accountability for GMA AI products throughout product lifecycle. Solutions may range from...SuggéréTemps plein
- ...Join Roche where every voice matters. The Position Drive impactful real-world evidence generation. In this role as a Medical Affairs Operations Leader you will design and coordinate non-interventional studies providing operational leadership across complex...SuggéréTemps pleinRemote job
- To grow and strengthen our Editorial team at the Headquarter of MDPI, we are looking for recent graduates who are eager to stay engaged with academia and contribute to making scientific research widely accessible by helping researchers worldwide publish their latest results...SuggéréTemps pleinHoraire flexible
- ...Ayus Medical Basel is part of the Ayus Medical Group a global collective redefining medical relief as a new field of care. Together within a multiprofessional team we develop structures and procedures to specifically address the physiological and biochemical burdens...Suggéré
- ...our client, a dynamic international biotechnology company focused on clinical-stage development, we recruit a: Senior/Executive Medical Director - Clinical Developement Location: Based in Basel, Switzerland / Remote model Responsibilities: Lead Clinical...SuggéréRemote job
- ...everyone has access to healthcare today and for generations to come. Join Roche where every voice matters. The Position We are Medical Affairs. We are transforming Medical practice together. We are accountable for the medical outside-in perspective at Roche and for...SuggéréTemps plein
- ...dedicated and passionate people united by a drive to achieve together. You will be a principal legal advisor to the Head International Medical Affairs (IMA) and the IMA Leadership Team on medical strategies plans and activities of Medical Affairs across International Region...Temps plein
- ...Engineering Intern you will gain hands-on experience in the development and characterization of components for one of our flagship medical devices. Working alongside experienced mentors youll validate theoretical assumptions explore material and process design and contribute...Temps pleinAu printempsRemote jobRecrutement immédiat
- ...Job Description Summary This is an opportunity to partner closely with the Global Head Medical Affairs and play a central role in driving what matters most across Global Medical Affairs (GMA). Youll operate at the heart of the organisation bringing focus momentum and...Temps plein
- ...Key responsability Keep overview over processes and agreements involving medical information at HQ affiliates as well as service providers contracted Stay informed about Medical Information related systems available General MedInfo tasks and -inquiry management...Temps pleinRemote job
- ...can step in as needed—mostly during the day or evenings—to watch over her, so I can rest (currently dealing with a serious family medical emergency). Reliability matters to us, as does a warm personality and clear communication. First aid knowledge is a plus, and experience...Quart du jour
- ...appointed from 01.02.2027 or by arrangement The Faculty of Medicine of the University of Basel is looking to fill the position of a Professor of Anatomy. The Medical Faculty of the University of Basel is seeking to fill a Professorship in Anatomy starting January 2027 ...
- ...Accommodation: Novartis is committed to working with and providing reasonable accommodation to all individuals. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the recruitment process, or in order to receive more...Temporaire
- ...accommodation: Novartis is committed to working with and providing reasonable accommodation to all individuals. If, be-cause of a medical condition or disability, you need a reasonable accommodation for any part of the recruitment process, or in order to receive more detailed...Travail posté
- ...Proclinical is seeking a Packaging Engineer to support packaging development and industrialization for combination products within the medical device and pharmaceutical sectors. This role involves working on diverse projects, collaborating with stakeholders, and...
- ...accommodation: Novartis is committed to working with and providing reasonable accommodation to all individuals. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the recruitment process, or in any order to receive...
- ...accommodation: Novartis is committed to working with and providing reasonable accommodation to all individuals. If, because of a medical condition or disability, you need a reasonable accommodation for any part of the recruitment process, or in any order to receive...
- ...consider Roche. We rank among the worlds foremost corporations providing innovative healthcare solutions, continuously investing in medical research and development. By preventing, diagnosing and treating a range of health disorders, our products and services have...Temps pleinEmploi postdocHoraire flexible
- ...date: Sept 1st, 2025 or by agreement limited to 1 year Ihr Arbeitsumfeld We are currently seeking an Epidemiologist with a strong medical background to join our dynamic research team. Our work involves conducting clinical trials of interventions with potential public ....
- ...a new myelofibrosis treatment, ensuring alignment with hematology objectives. Orchestrate cross‑functional collaboration across medical, regulatory, access, commercial, and supply teams to enable seamless execution. Drive launch readiness across priority markets,...
- ...Executive Director reports to the Renal International Disease Area Head and works closely with cross‑functional partners in Development, Medical Affairs, Value & Access, and Customer & Market Activation to ensure aligned and effective strategy implementation. The role also...Travail posté